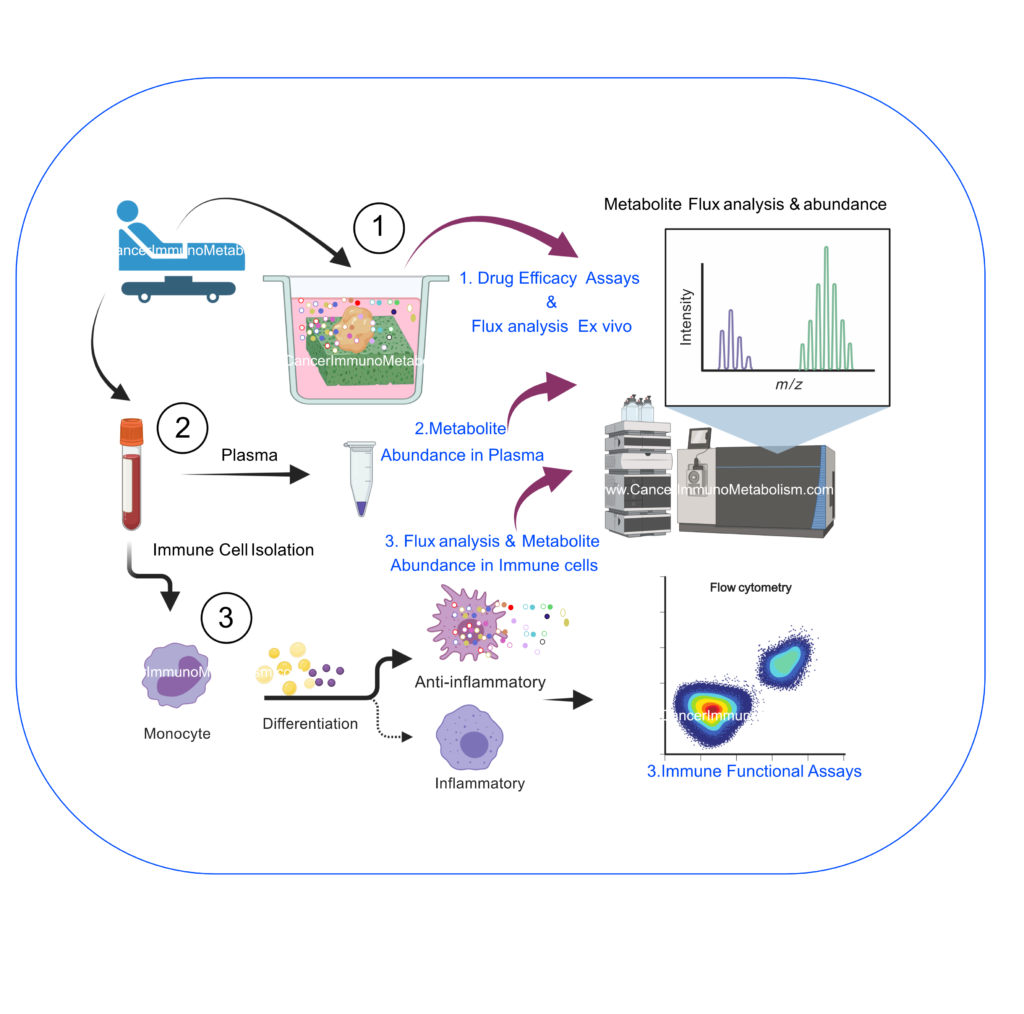
In higher multicellular organisms, each cell type displays a unique metabolic signature, forcing the intercellular exchange of metabolites. These shared nutrients can serve as building blocks for structural macromolecules and/or signaling mediators. In tumor microenvironment, we and others demonstrated that immune and tumor cells exchange unique metabolites either during tumor progression or treatment (El-Kenawi, et al, 2021, El-Kenawi, In Prep). Deciphering the detailed mechanism of this symbiotic relationship is crucial to understand mechanisms of drug resistance in cancer. In my current work, I utilize liquid chromatography (LC) with high-resolution mass spectrometry (HRMS) and other multi-omic approaches to identify novel metabolic interactions in cancer.
How Cancer Cells Persist during Treatment

Many efficacious anti-cancer treatments aim to eradicate the majority of cancer cell population. However, resistance almost inevitably occurs due to development of therapy-tolerant persister cells. We use time-lapse imaging with longitudinal DNA/RNA sequencing and metabolomics to decipher the strategies of molecular evolution of cancer cell persisters. We show that cancer cell persisters adopt metabolic non-genetic mechanisms to evade cell death and activate cell proliferation programs. Discovering these metabolic strategies in cancer cell persisters is crucial to prevent cancer recurrence.
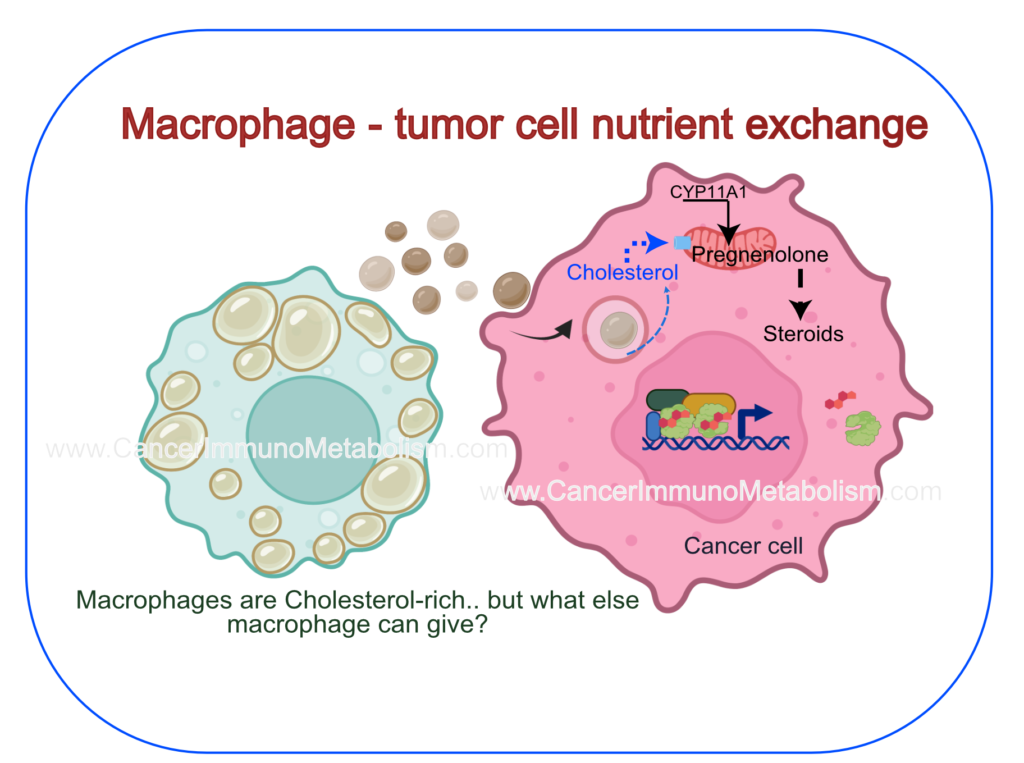
Scavenging Cholesterol Comes at a Cost
Preclinical evidence has shown that progressing cancers rely on cholesterol to support cell proliferation, plasma membrane architecture and steroid biosynthesis. Cholesterol can be synthesized de novo or acquired from extracellular sources. Since the process of cholesterol synthesis is energetically costly, malignant cells evolved to scavenge extracellular cholesterol from extrinsic sources such as other cells. However this comes at a cost by exposing other metabolic liabilities. We aim to define the coordinated transcriptional and metabolic adaptations to high level of cholesterol in macrophage and tumor cells to identify novel therapeutic targets.