Category: Publications
El-Kenawi, et al., 2019, Cancer likes it sour!
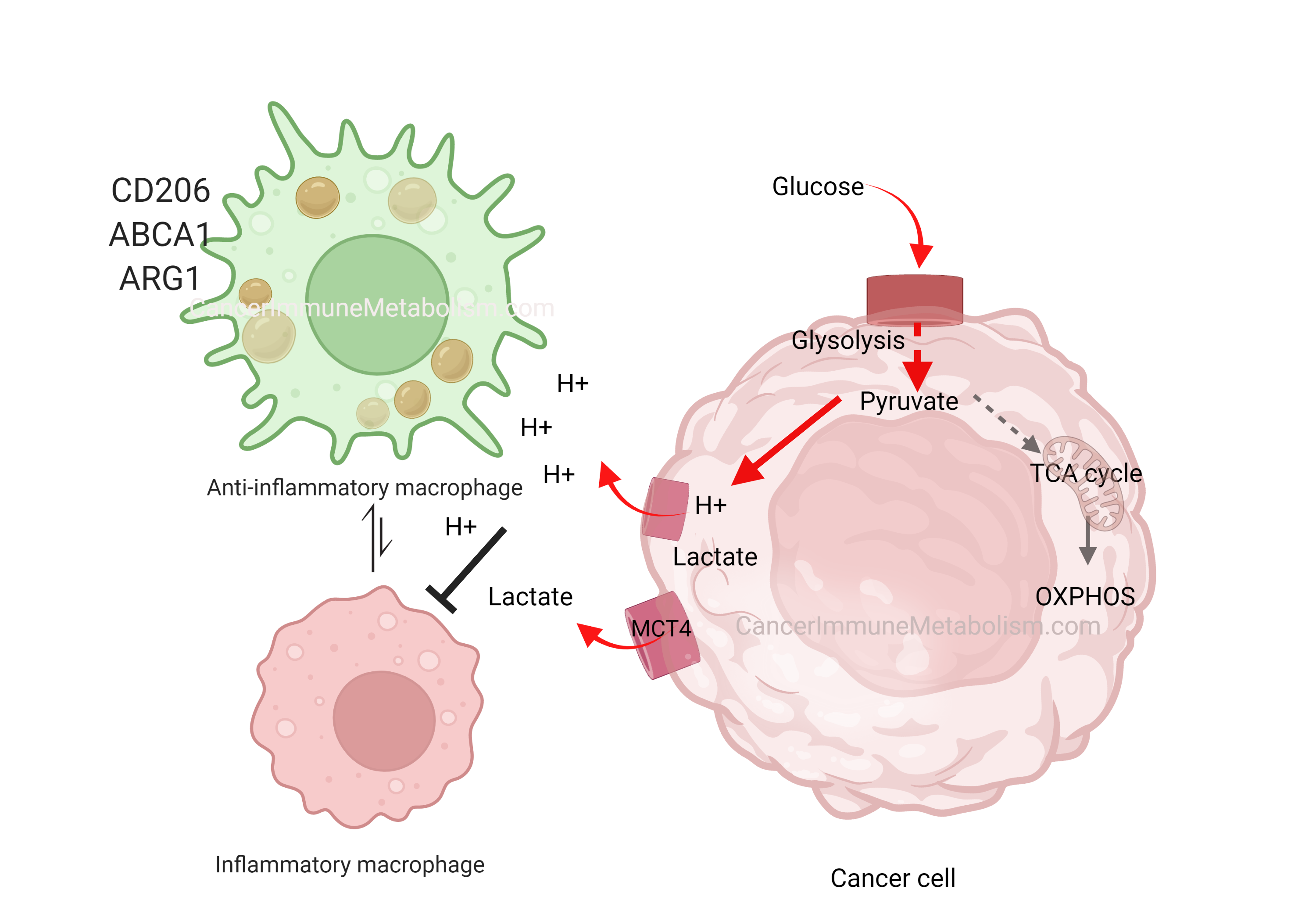
In his early work, the great Otto Warburg was the first to describe cancer cells’ preference to metabolize glucose into lactate even in the presence of oxygen, a phenomenon termed aerobic glycolysis. Although aerobic glycolysis is an inefficient way to generate cellular energy (producing 2 ATP molecules versus oxidative phosphorylation’s 36 ATP), it provides cancer cells with proliferative advantages because:
- It facilitates the uptake and incorporation of nutrients into the growing biomass (Reference)
- Metabolic products like hydrogen ions and lactate acidify the extracellular space, creating harsh conditions that kill normal cells while allowing tumor expansion (Gatenby and Gillies, 2004)
In El-Kenawi, et al., 2019, we demonstrated that acids released by cancer cells can polarize macrophages toward a pro-tumor phenotype. As versatile immune cells, macrophages adapt their function based on microenvironmental cues. By acidifying their surroundings, cancer cells effectively neutralize macrophages’ cytotoxic potential. This prostate cancer-induced macrophage reprogramming was marked by cytokine release and upregulation of CD206, ARG1, and the cholesterol transporter ABCA1—findings that motivated our subsequent investigation of macrophage cholesterol metabolism in later work.
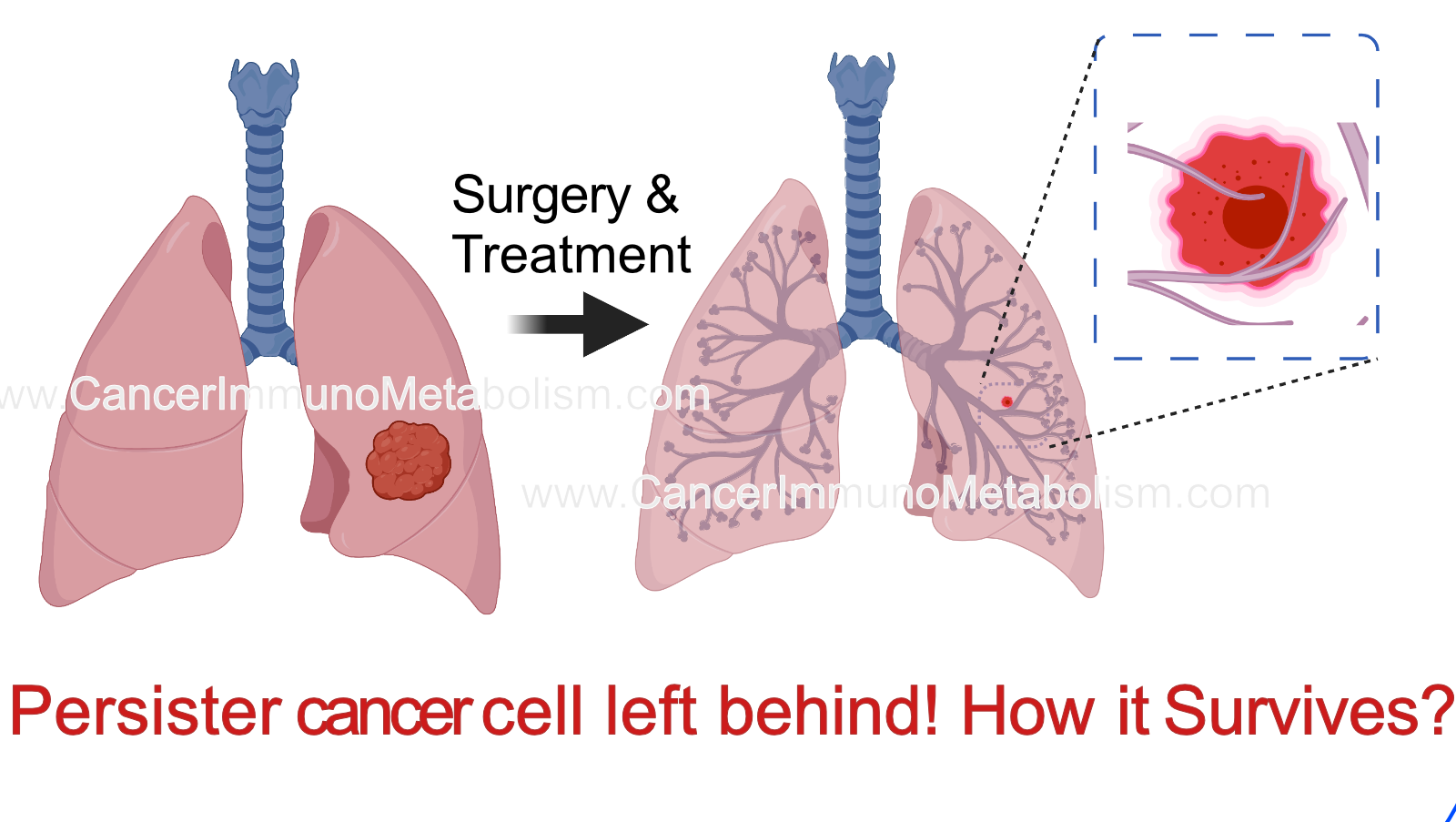
El-Kenawi et al., 2023 Persisters: the story of cells left behind!
Evolution of resistance is a major barrier to prolonged tumor control. Growing evidence suggests that resistance can be driven by few number of cells which remains after treatment, known as persisters or residual tumor cells. How these cells resist anti-cancer treatment? It depends on the way they die with or what we call “cell death pathways”!
There are many types of cell death pathway: such as apoptosis, necroptosis, pyroptosis and necrosis.
In this paper, we focus on mechanism which enable resistance to pyroptosis, a type of inflammatory cell death which usually occur in macrophages, a type of innate immune cells. To decipher the molecular mechanisms of resistance to pyroptosis, we combined time-lapse imaging with longitudinal DNA/RNA sequencing and metabolomics. We found that these persisters lap up methionine to counteract the detrimental impact of pyroptosis signaling on plasma membrane integrity. However, this will also increase the methylation capacity leading to various epigenetic changes.
Read More on the Cancer Research Website Here
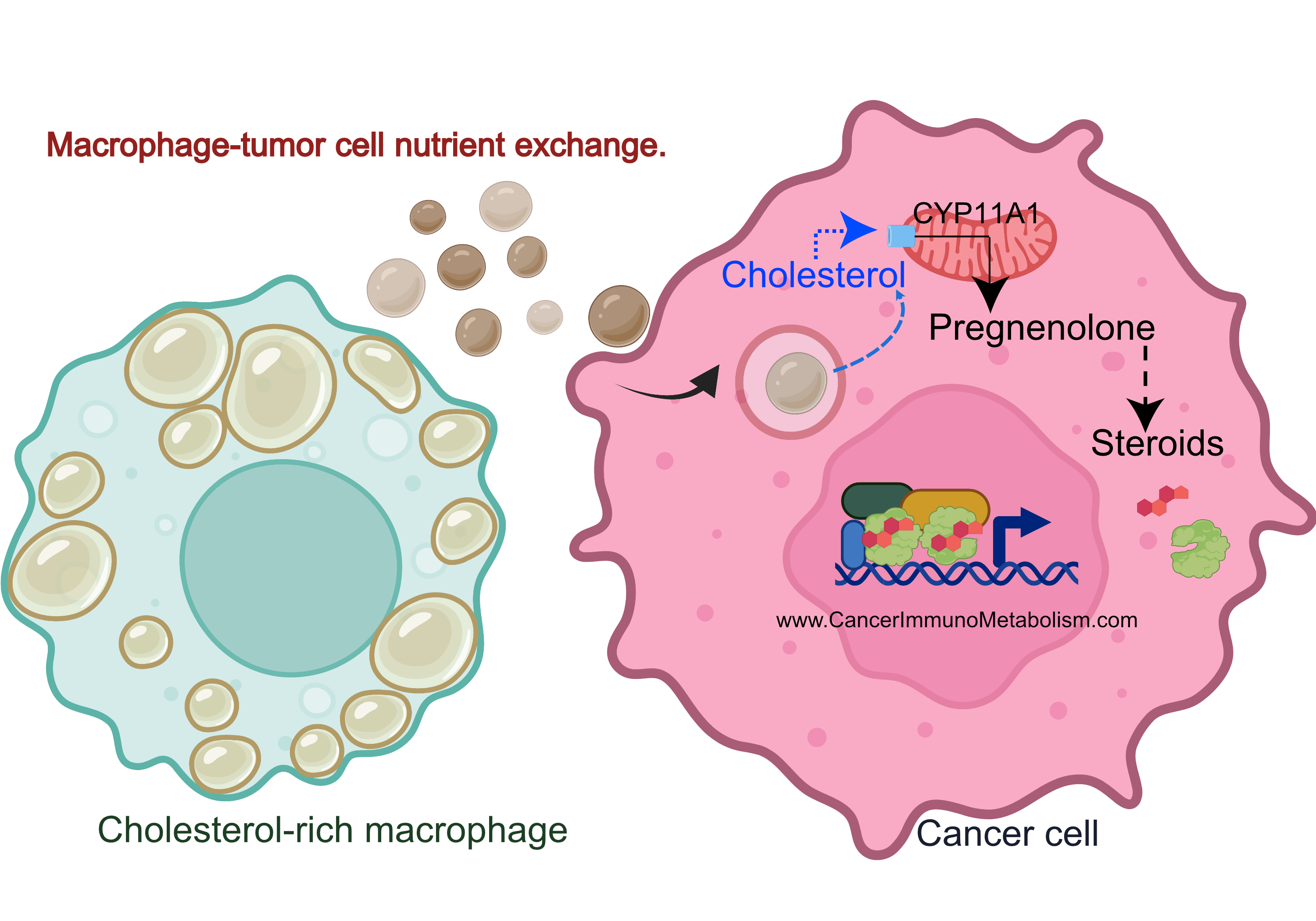
El-Kenawi et al., 2021, Macrophages feed cancer cells!
Do you remember my highlight from El-Kenawi, et al., 2019? During this project, I observed cholesterol accumulation in prostate cancer-associated macrophages, with acidity dramatically increasing intracellular lipids.
Why is this important?
Cholesterol is used to make the 5 main classes of steroid hormones.
Cancers like prostate, breast and ovarian cancers use steroidal hormones to proliferate. While therapies blocking these hormones are effective, resistance often develops when tumors increase cholesterol utilization for local steroid production.
Key Research Questions:
- Does macrophage activity regulate response to steroid hormone receptor therapies (e.g., androgen receptor antagonists)?
- Does cholesterol exchange play a role in therapeutic resistance?
In El-Kenawi et al., 2021, we employed:
to investigate macrophage cholesterol’s role in hormonal therapy response, validating findings with ex vivo patient-derived prostate cancer tissues.