Author: Asmaa El-Kenawi
SCAPING Inflammation!
By Krystal Villalobos-Ayala
Endothelial cells (EC) respond to a plethora of external stimuli (i.e. infection,
inflammatory cytokines) and with constant activation of EC can lead to atherosclerosis and
recruitment of inflammatory immune cells. The relationship between inflammation and
cholesterol homeostasis has only been studied in atherosclerosis, however, the mechanism of
action has not been elucidated.
Fowler et al, show the close relationship between inflammatory signaling (TNF-α→NF-
kB) and cholesterol homeostasis (SCAP-SREBP complex). The authors show that inflammatory
pathways and the cholesterol biosynthesis pathway is upregulated in cells upon TNF-α
introduction, which is all dependent on Rela (activator of NF-kB). TNF-α increases SREBP2
cleavage, which activates it, and cholesterol biosynthesis proteins are produced. The authors
then investigated step by step of how the cholesterol biosynthesis pathway is upregulated using
biochemical and genetic assays. The authors found that the TNF-α activation of SREBP2 in
cells is dependent both on NF-kB activation with Rela and its DNA-binding ability to increase the
production STARD10. STARD10 regulates trafficking of cholesterol and may be implicated in
reorganization of plasma membrane lipids which may affect cholesterol flux. The alteration of
accessible cholesterol is sensed by the SCAP/SREBP2 complex. The authors also show that
shuttling of the SCAP/SREBP2 complex from one cell compartment to the next (endoplasmic
reticulum to the Golgi) in TNF-α treated cells is essential in cleaving/activating SREBP2. An
activated SREBP2 is now able to produce the proteins involved in cholesterol biosynthesis.
Fowler et al, mechanistically characterized how inflammation affects cholesterol
homeostasis. Moreover, more studies need to be done in a model of chronic inflammation to
explore how SREBP2 activation affects endothelium vascular permeability, receptor signaling
and sensitivity to infections.
A selected paper highlight for Fowler et al. 2022 from a pool of research highlights,
Cancer Immunotherapy class of 2023
written by Krystal Villalobos-Ayala, University of South Florida PhD Program
Elucidating histone deacetylase 3 and its role in CD4 + T cell activation
By Sara Leahey
CD4 + T cells undergo a metabolic shift after activation, increasing cellular processes that make blasting and proliferation possible. A Class I histone deacetylase called histone deacetylase 3 (HDAC3) represses gene expression through the deacetylation of lysine residues on histones 3 and 4. HDAC3 has been shown to be a key regulator in gene expression for the T cell developmental pathway.
The authors worked on elucidating the role of HDAC3 in this metabolic shift of CD4 + T cells. The authors proceeded with a mouse model that uses the Cre recombinase system driven by the distal promoter of the lymphocyte-specific Lck, which results in HDAC3 being knocked out specifically in T cells. They’d found that the differentiation of CD4 + T cell subtypes are comparable in both the HDAC3-sufficient (WT) and HDAC3 knockout (KO) mice. When looking at total cell number, the HDAC3-deficient CD4 + T cells had reduced numbers compared to the WT cells, suggesting difference in proliferative capacity. Going further into this, they looked at the proliferation capacity of enriched CD4 + T cells from the HDAC3 KO mice and showed reduced viability and proliferative capacity in HDAC-deficient CD4 + T cells. The authors concluded that HDAC3 is necessary for CD4 + T cell proliferation after activation.
The authors decided to investigate mTORC1’s involvement in the metabolic switch and how it may be related to the decrease of proliferation and blasting in HDAC3-deficient CD4 + T cells. Interestingly, downstream signaling events in the mTORC1 pathway were decreased in the CD4 + T cells isolated from HDAC3 KO mice, along with decreased oxygen consumption rate (OCR). These data helped explain how HDAC3 may be affecting the proliferation of CD4 + T cells through metabolic reprogramming. To dive more into the mechanism of this metabolic reprogramming, the authors explored lipid synthesis, post-T-cell activation. It was found that not only is blasting reduced, the cholesterol levels also decreased in the HDAC3 KO CD4 + T cells, compared to the WT CD4 + T cells. The authors discovered that genes involved in cholesterol efflux, ABCA1 and ABCG1, were increased in the HDAC3 KO CD4 + T cells.
Further investigation showed that Abca1 and Abcg1 genes are hyperacetylated after loss of HDAC3, suggesting a role of HDAC3 in repressing these genes through deacetylation and thus preventing cholesterol efflux during CD4 + T cell activation and proliferation. These findings are important in our understanding of how changes in metabolic reprogramming can negatively affect T cell activation and proliferation.
A highlight and Commentary by Sara Leahey (Graduate Student) for Wilfahrt, et al., 2021: Histone deacetylase 3 represses cholesterol efflux during CD4+ T-cell
activation. eLife
How to constructively critique a manuscript: Literature Review
by El-Kenawi, A.*
Objectives:
To teach graduate students how to come with a constructive peer-review for manuscripts.
To teach students how to write a lay abstract or a highlight for a published paper.
Plan:
- Individual effort: Each student will prepare a lay abstract (400 words, also known as
research highlight) ahead of the class to summarize the paper. Lay abstracts are a way
to convey the summary of scientific work to audience with very low experience in the
subject. Examples of these audience are scientists in other field, public, patient
advocates and funding agencies administrators. The best two lay abstracts will be
published in this blog with the student’s name highlighted (only with the students’ permission).
Examples of research highlights here
Tip: don’t use complicated terms. Think of analogy and applications which can benefit
the public. - Team-effort: Students can work together on brief introduction to the paper (15 min) that
include background, important figures and their conclusion for the paper. Each student
can present a section or 1-2 slides. - We will then open discussion addressing the following (during class time):
- -What main points the authors were trying to make?
- -Are all the results obtained consistent with the hypothesis being tested? If not please
give an example. - -What sort of evidence (additional suggested experiment/ or a modification of already
reported experiment) would make the authors’ case stronger? - -What sort of evidence would argue against the authors story? You can cite other papers.
- -Can you find over- statements in the text that is not supported by data? In other words,
did the authors provide substantive support for their position? Which conclusions are
directly drawn from the analysis of the results, and which are more speculative? - -Can you suggest rephrasing to make these speculative statements more precise
reflecting the actual data presented? This can be used by the reviewer as a feedback for the authors instead of asking for new experiments. - -What case would a “skeptical scientist” make against the authors’ interpretation of their
results? - -Fun: ” Wrong answers only” Can you come with an overstatement or wrong conclusion of any of the figures?
- -After answering these question, can you check the peer-review publicly posted report if available. eLife posts peer-review report. please suggest other journals with similar policies.
Research Article Assignments:
- 2022
Wilfahrt, et al., 2021: Histone deacetylase 3 represses cholesterol efflux during CD4+ T-cell
activation. eLife
Check out Sara Leahey commentary here. - 2023
Fowler et al., 2022: Inflammatory stress signaling via NF-kB alters accessible cholesterol to
upregulate SREBP2 transcriptional activity in endothelial cells. eLife
Check out Krystal Villalobos-Ayala commentary here.
*I give this class as a part of Cancer Immunotherapy, Immunology PhD Program.
The Fantastic BH4 matters in T cells
Tetrahydrobiopterin (BH4) is an essential cofactor in the biosynthesis of molecules regulating neurotransmission and vascular tone. In a recent publication, Cronin and colleagues discovered a novel role of BH4 in regulating T cell immunity.
Cells generate BH4 through de novo synthesis catalyzed mainly by GTP cyclohydrolase I (GCH1) and sepiapterin reductase (SPR). Through modulating the de novo synthesis pathway, Cronin et al. addressed the roles of BH4 in T cells. They found out by using genetically engineered mice that loss of GCH1 in T cells abrogates proliferation without affecting T cell development or activation markers. Through using models of inflammatory and autoimmune diseases, the authors showed that lowering BH4 levels through genetic ablation of GCH1 or pharmacological inhibition of SPR downgrades the severity and signs of injury. They attributed the effect of GCH1 loss to changes in iron redox status, which in turn controls the mitochondrial function and energy production, required for T cell proliferation. Supplementing T cells with BH4 or the BH4 precursor sepiapterin, could reverse the impact of GCH1 deficiency. As expected, the ability of BH4 to expand the effector T cell population was sufficient to reduce tumor burden in T cell-dependent cancer models. Collectively, the study established a role of BH4 in T cell proliferation involved in a range of pathological conditions.
For future studies and translational applications, BH4 supplementation could be combined with adoptive cell transfer and immunotherapy to enhance their efficacy. In addition, data presented could provide new hypotheses to the mechanisms of methotrexate, an antineoplastic and anti-inflammatory drug. Since methotrexate strikingly decreases BH4 levels, it is unknown whether this could adversely affect T cells in cancer patients treated with methotrexate. In such case, exogenous BH4 may provide a worthwhile benefit. Overall, this study provides tremendous opportunities to improve the efficacy of current immunotherapeutic and anti-cancer treatment modalities.
A highlight and Commentary by Asmaa El-Kenawi for Research Article:
Cronin SJF, Seehus C, Weidinger A, Talbot S, Reissig S, Seifert M, et al. The metabolite BH4 controls T cell proliferation in autoimmunity and cancer. Nature. 2018;563(7732):564-8.
Asmaa El-Kenawi, PhD
I am Pharmacy educator and an immune cell metabolism enthusiastic. I am obsessed with applying Physical Sciences perspectives and approaches to cancer therapy.
Outside the lab, I like to paint, travel and try new authentic food recipes. I also like watching soccer games. I am a mother of two boys.

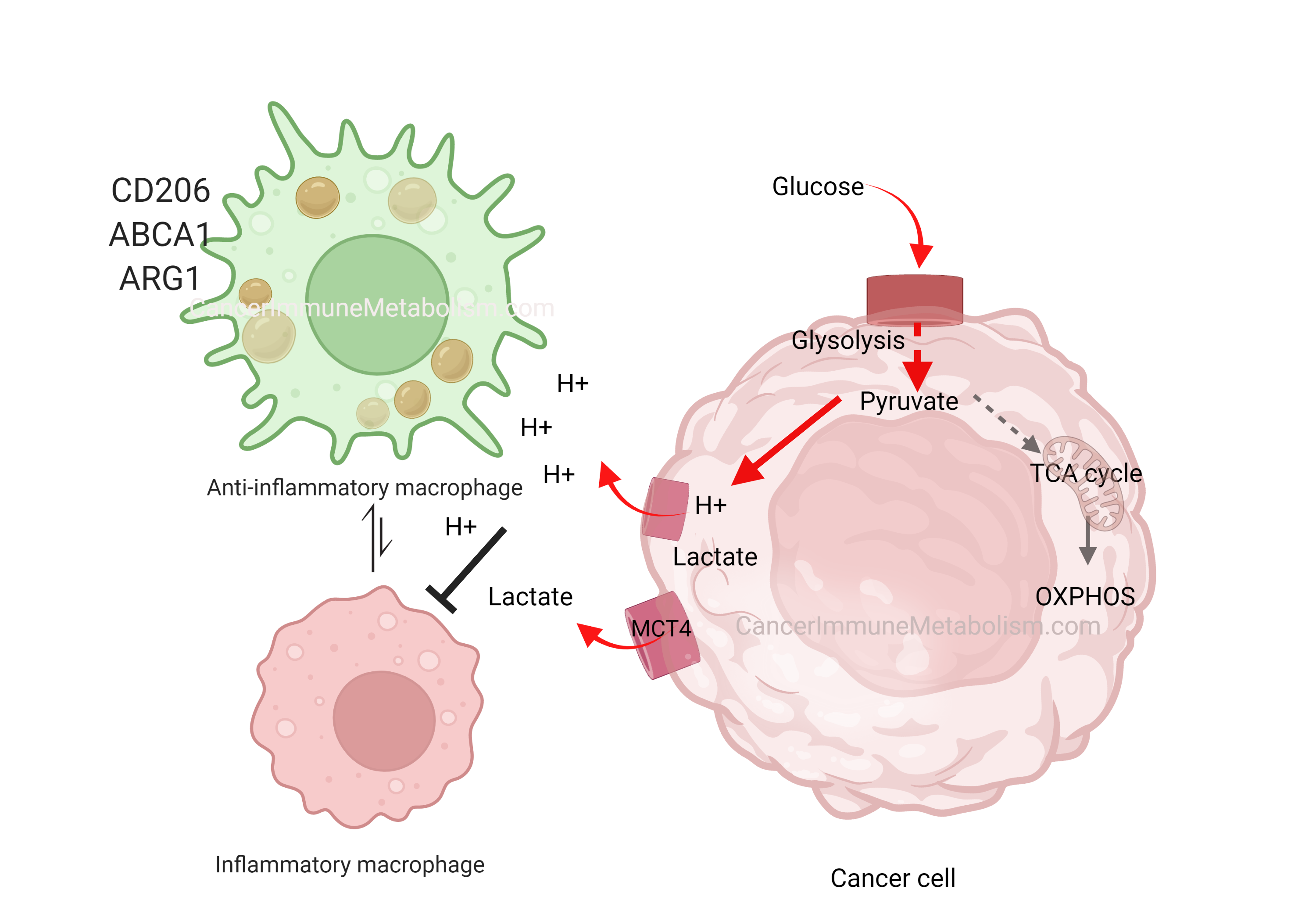
El-Kenawi, et al., 2019, Cancer likes it sour!
In his early work, the great Otto Warburg was the first to describe cancer cells’ preference to metabolize glucose into lactate even in the presence of oxygen, a phenomenon termed aerobic glycolysis. Although aerobic glycolysis is an inefficient way to generate cellular energy (producing 2 ATP molecules versus oxidative phosphorylation’s 36 ATP), it provides cancer cells with proliferative advantages because:
- It facilitates the uptake and incorporation of nutrients into the growing biomass (Reference)
- Metabolic products like hydrogen ions and lactate acidify the extracellular space, creating harsh conditions that kill normal cells while allowing tumor expansion (Gatenby and Gillies, 2004)
In El-Kenawi, et al., 2019, we demonstrated that acids released by cancer cells can polarize macrophages toward a pro-tumor phenotype. As versatile immune cells, macrophages adapt their function based on microenvironmental cues. By acidifying their surroundings, cancer cells effectively neutralize macrophages’ cytotoxic potential. This prostate cancer-induced macrophage reprogramming was marked by cytokine release and upregulation of CD206, ARG1, and the cholesterol transporter ABCA1—findings that motivated our subsequent investigation of macrophage cholesterol metabolism in later work.
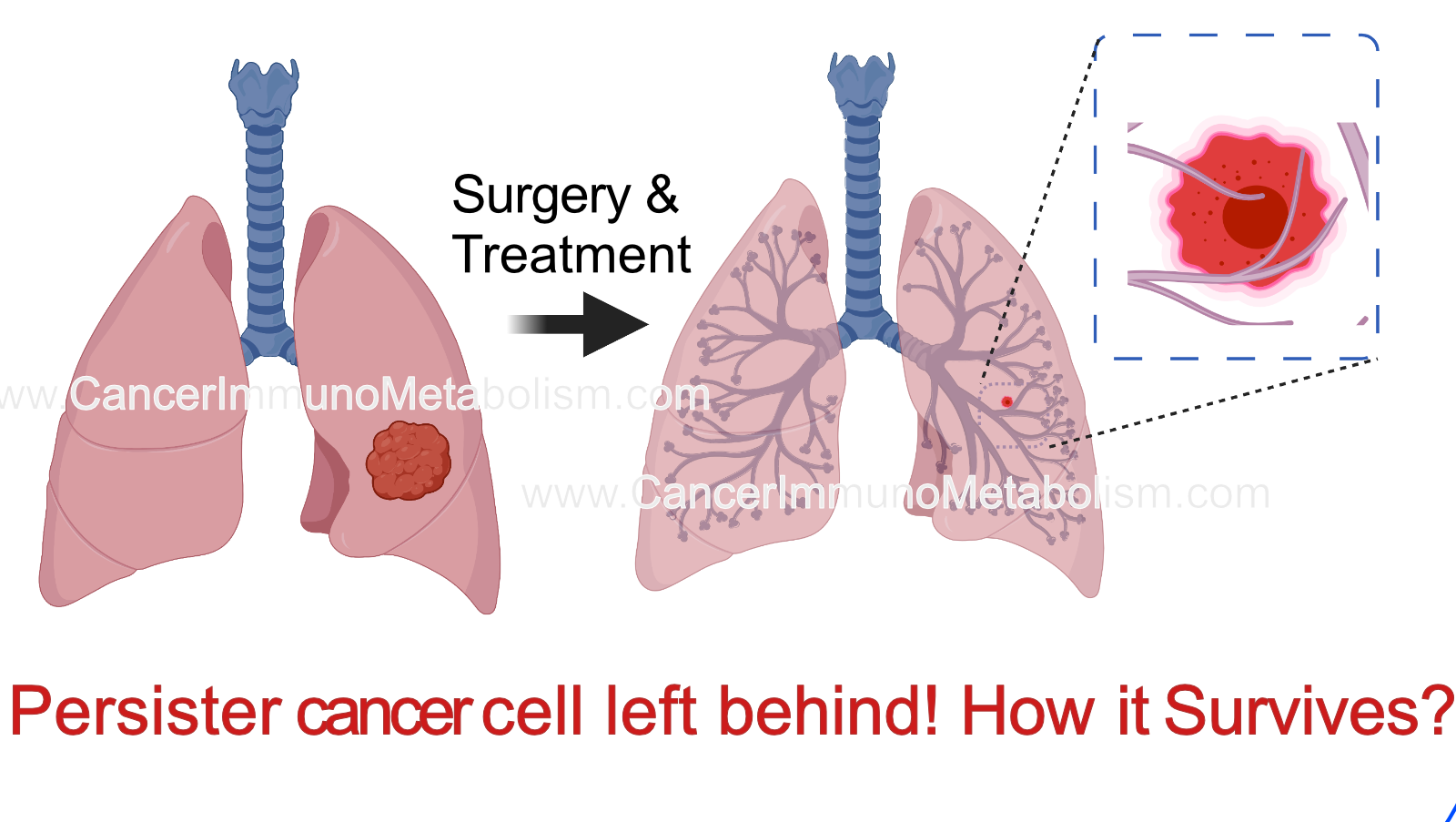
El-Kenawi et al., 2023 Persisters: the story of cells left behind!
Evolution of resistance is a major barrier to prolonged tumor control. Growing evidence suggests that resistance can be driven by few number of cells which remains after treatment, known as persisters or residual tumor cells. How these cells resist anti-cancer treatment? It depends on the way they die with or what we call “cell death pathways”!
There are many types of cell death pathway: such as apoptosis, necroptosis, pyroptosis and necrosis.
In this paper, we focus on mechanism which enable resistance to pyroptosis, a type of inflammatory cell death which usually occur in macrophages, a type of innate immune cells. To decipher the molecular mechanisms of resistance to pyroptosis, we combined time-lapse imaging with longitudinal DNA/RNA sequencing and metabolomics. We found that these persisters lap up methionine to counteract the detrimental impact of pyroptosis signaling on plasma membrane integrity. However, this will also increase the methylation capacity leading to various epigenetic changes.
Read More on the Cancer Research Website Here
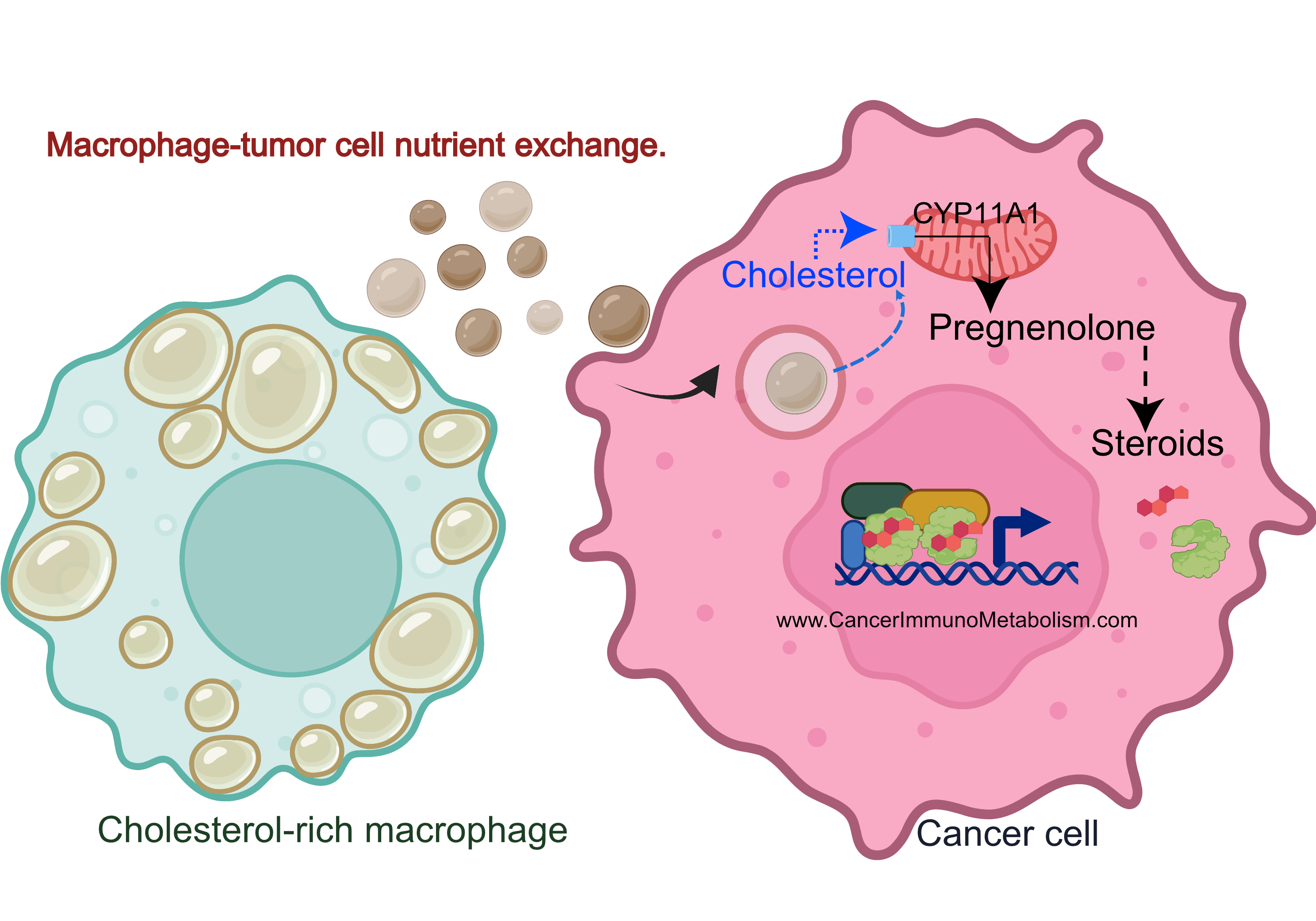
El-Kenawi et al., 2021, Macrophages feed cancer cells!
Do you remember my highlight from El-Kenawi, et al., 2019? During this project, I observed cholesterol accumulation in prostate cancer-associated macrophages, with acidity dramatically increasing intracellular lipids.
Why is this important?
Cholesterol is used to make the 5 main classes of steroid hormones.
Cancers like prostate, breast and ovarian cancers use steroidal hormones to proliferate. While therapies blocking these hormones are effective, resistance often develops when tumors increase cholesterol utilization for local steroid production.
Key Research Questions:
- Does macrophage activity regulate response to steroid hormone receptor therapies (e.g., androgen receptor antagonists)?
- Does cholesterol exchange play a role in therapeutic resistance?
In El-Kenawi et al., 2021, we employed:
to investigate macrophage cholesterol’s role in hormonal therapy response, validating findings with ex vivo patient-derived prostate cancer tissues.